High impact discoveries for the real world
Our lab is driven by the pursuit of discoveries that matter — insights into gene regulation, metabolic flexibility, and evolution that reveal fundamental principles with wide-reaching applications. From understanding the genetic basis of biological flexibility and adaptive responses, to uncovering natural mechanisms of disease and aging resilience, our research bridges neuroscience, genetics, metabolism, genomics, data science, evolution, and ecology. We aim to generate knowledge that not only advances science but opens new paths toward improving human health.
Comparative Genomics: Uncovering the Genetic Code for Biomedical Superpowers
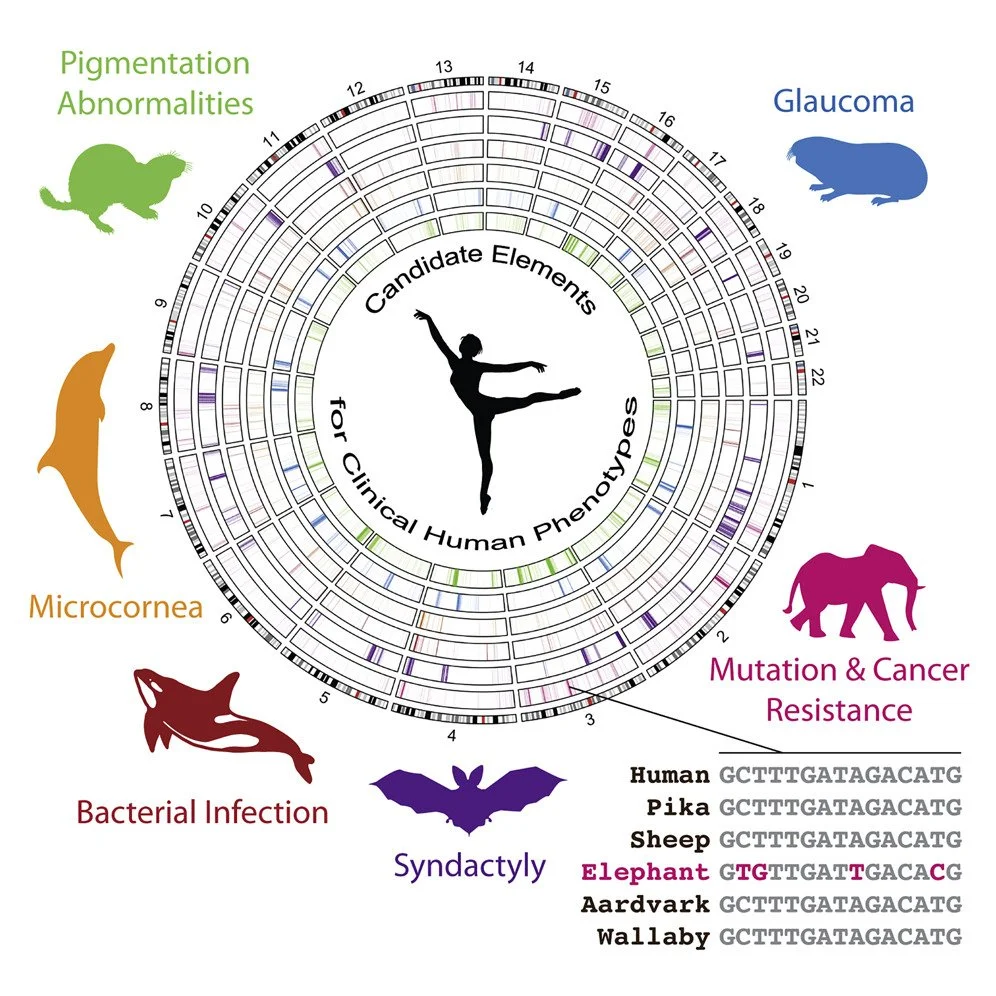
Our lab harnesses the power of comparative genomics to identify conserved and divergent gene regulatory elements across species with remarkable biological traits—such as hibernation, cancer resistance, and metabolic flexibility. These species, including hibernating mammals and long-lived, tumor-resistant elephants, exhibit natural adaptations that resemble solutions to major biomedical challenges.
By analyzing conserved noncoding regions of the genome that have undergone accelerated evolution in species with exceptional traits, we pinpoint candidate cis-regulatory elements (CREs) that may control complex phenotypes like obesity, torpor, and mutation resistance. We integrate these insights with functional genomics tools—including CRISPR-based deletions, RNA-Seq, ATAC-Seq, and 3D chromatin mapping—to investigate how these elements regulate gene networks in the hypothalamus and other key tissues.
Key discoveries include:
Hundreds of hibernation-linked CREs enriched in topologically associated domains (TADs) like the FTO-Irx3 locus, a region strongly associated with human obesity risk.
Distinct gene circuits and epigenetic modules underlying metabolic state transitions, such as those seen in fasting, refeeding, and torpor.
Accelerated cis-elements in cancer-resistant species like elephants that target DNA repair pathways, offering clues for therapeutic innovation.
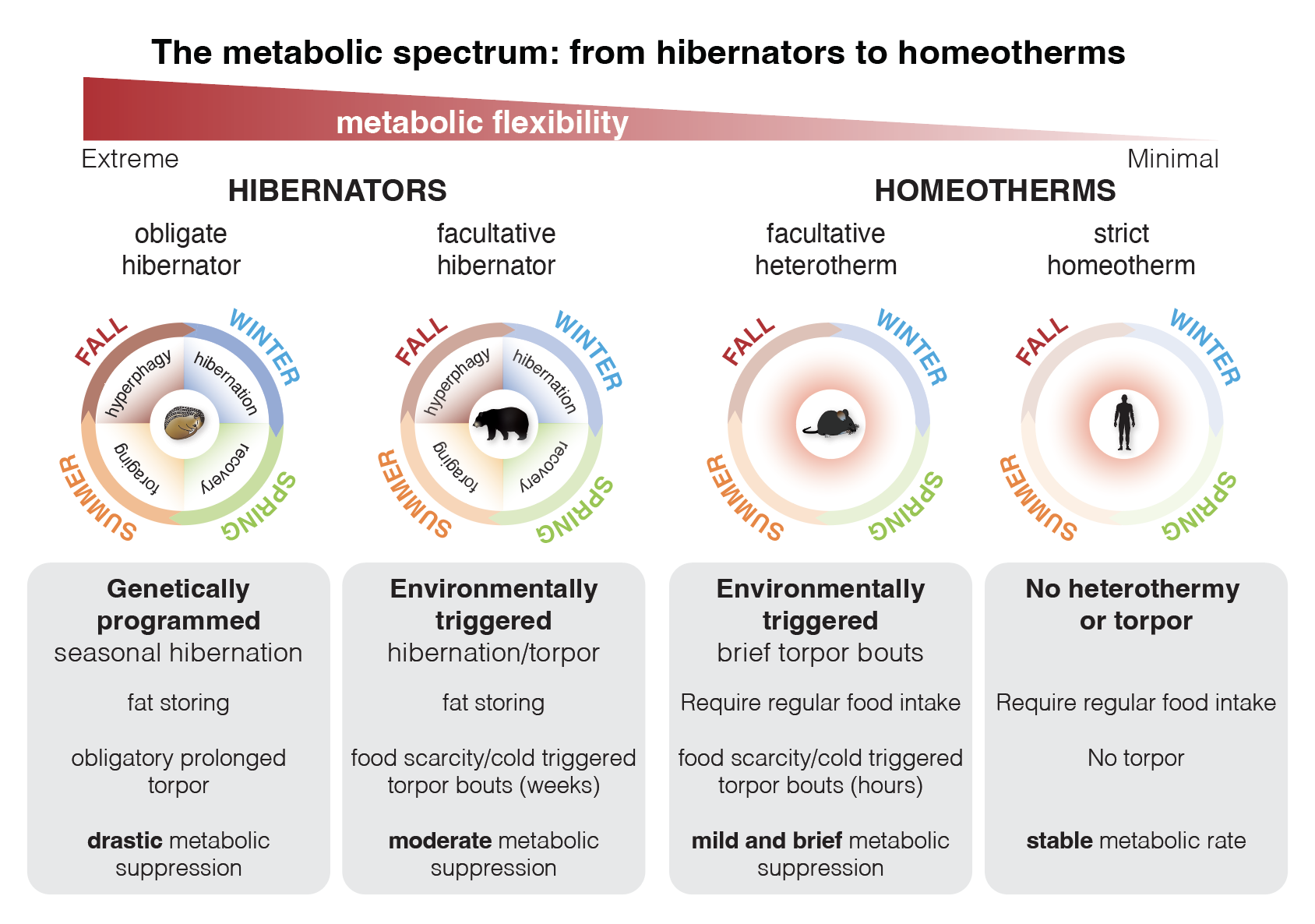
Hibernation has evolved independently in multiple lineages and we found evidence that it involves loss-of-function changes to CREs conserved in non-hibernators.
Deletions of individual hibernation-linked CREs in mice (homeotherms) causes changes to gene expression, fasting responses, metabolic phenotypes, and age-related behavioral changes.
Our goal is to translate these natural adaptations into mechanistic insights and therapeutic targets, revealing how evolutionary biology can inform the treatment of human diseases such as cognitive decline, Alzheimer’s disease, obesity, cancer, and metabolic disorders.
Understanding the mechanisms of resilience and recovery.
Our lab investigates how the genome orchestrates resilience and recovery in the face of extreme physiological stress—such as extended fasting, metabolic shutdown, and cellular damage. By focusing on natural models of adaptation like hibernation and refeeding after fasting, we aim to identify genetic programs and regulatory elements that promote repair, reactivation, and homeostasis.
We have discovered metabolically responsive cis-regulatory elements (CREs) that act as control switches for key genes in the hypothalamus—particularly during the transition from starvation back to feeding. Using RNA-Seq, ATAC-Seq, PLAC-Seq, and genetic knockouts, we uncovered dynamic transcriptional programs that activate during refeeding, regulating metabolism, inflammation, chromatin remodeling, and neuroprotection.
In parallel, we study random allelic expression (RAE), a widespread phenomenon in which only one parental allele of a gene is expressed in a given cell or tissue. Our work in both humans and mice reveals that RAE affects thousands of genes, is influenced by metabolic states, and may serve as a mechanism for cellular diversity and adaptive flexibility—especially during recovery from stress.
By integrating comparative genomics with single-cell and functional assays, we are also exploring how hibernating species evolved unique genomic architectures for resilience—through conserved, accelerated CREs that tune metabolic and neural responses during deep torpor and rewarming.
Key discoveries include:
Identification of hypothalamic CREs that activate gene programs specifically during refeeding after extended fasting.
Discovery that refeeding triggers dramatic, genome-wide chromatin and transcriptional changes—including sustained shifts in metabolism, stress response, and neuroprotection.
Characterization of “hibernation-linked” CREs with functional impacts on torpor, metabolism, and weight regulation in mice.
Demonstration that deletion of individual CREs rewires expression of genes like Fto, Irx3, and Irx5, altering behavioral and metabolic responses.
Genome-wide mapping of random allelic expression (RAE) in human and mouse tissues, revealing its prevalence and connection to rapidly evolving genes and adaptive processes.
Evidence that RAE impacts key genes linked to age-related diseases and may create cellular mosaics that buffer against environmental stress.
Together, these studies uncover a network of gene regulatory mechanisms that govern recovery and resilience, with broad implications for aging, disease resistance, and metabolic therapies.
Neural and Gene Regulatory Mechanisms of Behavioral Flexibility and Decision Making
Our lab is uncovering how the brain and genome coordinate to produce flexible, adaptive behavior in complex naturalistic environments. We focus on identifying the neural circuits and gene regulatory mechanisms that govern decision making, foraging strategies, and behavioral transitions—especially as animals adapt to changing internal states and external contexts.
Using a combination of behavioral modeling, genome engineering, and large-scale neural recordings, we dissect how genes and neural ensembles control moment-by-moment decisions and behavioral structure. We develop unsupervised computational methods to decompose naturalistic foraging behaviors into discrete, reproducible stereotyped behavioral sequences that serve as the building blocks of complex patterns.
We are particularly interested in how different metabolic states reshape both gene regulatory programs and behavioral expression. Our work has shown that changes in metabolic condition can shift behavioral stereotypy, reconfigure decision modules, and alter the expression of genes involved in motivational circuits and adaptive control.
Our research also investigates the genetic basis of behavioral flexibility. We have focused on how maternally and paternally imprinted genes—particularly those involved in monoamine signaling like Th and Ddc—differentially influence behavior across development, sex, and environmental context. These parent-specific effects map onto distinct behavioral modules, revealing deep evolutionary logic in the control of decision-making behavior.
Most recently, we’ve expanded this work using Neuropixels in vivo electrophysiology to record from thousands of neurons across brain regions as animals perform context-dependent behaviors. We are uncovering neural ensembles and computational motifs that shift dynamically with context, internal state, and gene-specific manipulations.
Key discoveries include:
Development of DeepFeats, an unsupervised machine learning pipeline to decompose naturalistic foraging into over 200 reproducible behavioral modules.
Discovery that specific modules are probabilistically organized and genetically programmed, forming a modular architecture for complex behavior.
Identification of maternal versus paternal allele-specific effects of Th and Ddc on distinct behavioral modules, revealing sex-specific and context-dependent genetic control of decision sequences.
Demonstration that behavioral modules are differentially expressed depending on metabolic state (e.g., fed vs. fasted), revealing internal-state gating of decision programs and influencing behavioral stereotypy.
Demonstration that deleting a single Hibernation-linked CRE can affect the expression of specific foraging behavior modules.
Ongoing work with Neuropixels recordings to identify large-scale neural ensembles in the hypothalamus, midbrain, and cortex that encode behavioral modules and dynamically adapt to context and physiological state.
Integration of behavioral, genetic, and neural data to build a multi-level model of decision-making systems—linking cis-regulatory elements to neural computations and behavior.
Together, this work builds a framework for understanding how genetic and neural systems cooperate with metabolic state to drive flexible or stereotyped behavior—offering new insights into psychiatric disorders, addiction, brain aging, and behavioral evolution.
Foundational Deep Learning Models for Brain Gene Regulation and Behavioral Adaptation
Our lab is developing a new deep learning models to decode the structure and logic of gene regulation and behavior across species. These foundational models aim to capture the complexity of cis-regulatory code, predict gene expression dynamics, and model the compositional structure of natural adaptive behaviors in response to internal states and environmental challenges.
We are building cross-species frameworks that integrate genomic sequences, epigenomic data, and 3D chromatin architecture to learn generalizable rules governing cis-regulatory element function and cell type genetic programs. These models are designed to uncover conserved and divergent regulatory strategies that shape metabolic, behavioral, and physiological traits—particularly in species with extreme adaptations such as hibernation, extended fasting, and metabolic suppression.
In parallel, we are training deep neural networks on large-scale video and neural activity data from naturalistic behavior paradigms. These models aim to learn latent structure in decision-making and behavioral flexibility, capturing how animals compose adaptive actions across contexts, developmental stages, and genetic backgrounds.
Our goal is to develop interpretable AI systems that link DNA to phenotype and neural dynamics to behavior, providing a unifying framework for understanding biological intelligence and resilience.
Emerging research directions include:
Cross-species deep learning models that predict regulatory element activity from DNA sequence and evolutionary history.
Models that uncover adaptive behavior sequences from latent state transitions due to context shifts, mutations, or metabolic changes.
Hybrid frameworks that combine regulatory code models with behavioral structure models to explore how genetic changes reshape organism-level adaptations.
This research represents a convergence of genomics, neuroscience, and artificial intelligence, laying the foundation for predictive models of biological function that scale from base pairs to behavior.
Operation Warp Speed for Cancer - Using Lessons from Evolution to Improve Cancer Care
We're decoding how evolution has built resilience into living systems—from hibernating mammals that suppress metabolism without damage, to allele-specific gene expression that diversifies cellular function. By uncovering the genomic and neural mechanisms that enable organisms to survive extremes, we're identifying new strategies for cancer therapy. In collaboration with leaders at the Moffitt Cancer Center and Huntsman Cancer Institute, our goal is to move beyond killing cancer cells to managing cancer as an adaptive ecosystem—using insights from nature to design smarter, more durable interventions.
About the Free Uncharted Course
The Uncharted Course is a free, self-paced learning experience that introduces a bold new framework for cancer care. It teaches clinicians, scientists, and strategists how to rethink cancer as a dynamic, adaptive system—and how to use tools from systems biology, AI, and evolutionary theory to outmaneuver it. The course combines engaging video lessons, case studies, and downloadable resources designed to help you make a difference—whether you're at the bench, bedside, or boardroom.